Background:
Here we describe an online network of patients with cancer, clinicians and researchers, layered on top of a comprehensive real world database of treatments and outcomes, continuously updated in near real time, and initially focused on multiple myeloma.
Methods:
Patients with multiple myeloma register at www.all4cure.com and sign HIPAA release forms to provide access to all of their medical records from all of the institutions from which they have received care. From the medical records, a structured dataset of treatments and lab results is extracted by a myeloma-dedicated team for display on each patient's de-identified, personalized dashboard. A discussion panel allows more than 1200 participating patients, clinicians and researchers to post comments or questions regarding each patient's specific circumstances. For each patient, lines of therapy and responses are determined according to IMWG and published criteria (Blood [2015] 126: 921-922). Here we report initial results from the treatments and responses among patients with multiple myeloma and plasma cell leukemia.
Results:
A total of 493 patients includes 26 with monoclonal gammopathy of undetermined significance (MGUS), 51 with smoldering myeloma, 408 with multiple myeloma, and 8 with primary plasma cell leukemia. Among patients with multiple myeloma, 42 (10.3%) had antecedent MGUS and/or smoldering myeloma. The average age is 65.6 (+/- 9.7). 50.9% are male and 49.1% are female. 42 states in the U.S. and 8 other countries are represented. Two patients with multiple myeloma had not started treatment by the time of this report. The remaining 414 patients with multiple myeloma or plasma cell leukemia received 1 to 16 lines of therapy. Among the 414 patients that received at least one line of therapy, a remarkable 89 distinct first line therapies were administered. The most common first line therapies were lenalidomide, bortezomib, dexamethasone (n=92), cyclophosphamide, bortezomib, dexamethasone (n=50), lenalidomide, bortezomib, dexamethasone + autologous stem cell transplant + lenalidomide maintenance (n=41), lenalidomide and dexamethasone (n=29), and bortezomib and dexamethasone (n=22). 62 patients received a first line therapy that was not received by any other patient in our database. This variation was attributable to participation in clinical trials, variation in the availability of drugs over time and different geographic locations, and the very large number of distinct combinations associated with multi-agent regimens.
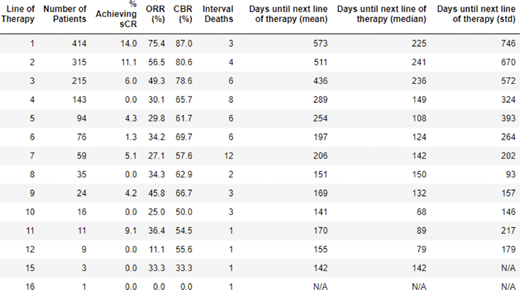
Seventy six percent of patients with multiple myeloma or plasma cell leukemia received a second line of therapy, 52% received a third line of therapy, 34% received a fourth line of therapy, 23% received a fifth line of therapy, and 18% received a sixth line of therapy. We observed a trend toward a decline in favorable responses with successive lines of therapy (Table 1) consistent with previous reports, with a correlation coefficient between the line of therapy and overall response rate of -0.74. Among the 76 patients who received 6 or more lines of therapy (247 lines of therapy in total), only 7 achieved stringent complete responses (sCRs), of which 5 were associated with a CAR-T therapy. Table 1 also shows a trend toward more rapid changes in treatment associated with later lines of therapy. 57 of 493 patients (12%) are deceased.
Conclusions:
Our findings reveal remarkable variation in the treatment of myeloma and provide a potentially valuable resource of up to date and current clinical features, prognosis, treatments, side effects, and outcomes of patients with myeloma in the real world.
Table 1. Summary of lines of therapy and outcomes across 414 patients with multiple myeloma and plasma cell leukemia. The percentages of patients achieving sCR, ≥ PR (overall response rate - ORR), and ≥MR (clinical benefit rate - CBR) are shown for each line of therapy. Also depicted is the interval mortality and the mean and median number of days between the beginning of a line therapy and the beginning of the next line of therapy.
Richter:Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Sanofi: Consultancy; X4 pharmaceuticals: Consultancy; Oncopeptides: Consultancy; Adaptive biotechnologies: Consultancy; Secura bio: Consultancy; Astra Zeneca: Consultancy; BMS: Consultancy; Karyopharm: Consultancy; Antengene: Consultancy. Chhun:All4Cure: Current Employment. Zheng:All4Cure: Current Employment. Piboonvaranggoon:All4Cure: Current Employment. Mallick:All4Cure: Current Employment. Wren:All4Cure: Current Employment. Nam:All4Cure: Current Employment. Lopez Barquilla:All4Cure: Current equity holder in private company. Blau:All4Cure: Current equity holder in private company. Cowan:Janssen: Consultancy, Research Funding; Abbvie: Research Funding; Cellectar: Consultancy; Sanofi: Consultancy; Bristol Myers Squibb: Research Funding. Bensinger:BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding, Speakers Bureau; Regeneron: Consultancy, Honoraria, Research Funding, Speakers Bureau. Kumar:Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Genecentrix: Consultancy; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Novartis: Research Funding; Merck: Consultancy, Research Funding; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Carsgen: Other, Research Funding; Dr. Reddy's Laboratories: Honoraria; Adaptive Biotechnologies: Consultancy; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; MedImmune: Research Funding; Tenebio: Other, Research Funding; BMS: Consultancy, Research Funding; Karyopharm: Consultancy; Cellectar: Other; Sanofi: Research Funding; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Kite Pharma: Consultancy, Research Funding. Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding. Anderson:Oncopep and C4 Therapeutics.: Other: Scientific Founder of Oncopep and C4 Therapeutics.; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium-Takeda: Membership on an entity's Board of Directors or advisory committees. Blau:All4Cure: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal